Navigating the complex landscape of medical device regulation is a formidable challenge for manufacturers worldwide. In particular, the European Union’s Medical Device Regulation (EU MDR 2017/745) has introduced stringent new requirements, fundamentally reshaping how devices are brought to market and maintained throughout their lifecycle. Among the most critical aspects of this regulation are the Essential Requirements (ERs), detailed in Annex I. These aren’t just suggestions; they are the bedrock of device safety and performance, and demonstrating meticulous adherence is paramount for market access and continued compliance.
For many organizations, the sheer volume and intricate nature of these requirements can feel overwhelming, leading to potential oversights, delays, and even non-compliance. This is precisely where a structured, comprehensive tool becomes indispensable. An Mdr Essential Requirements Checklist Template offers a clear, actionable framework, transforming a daunting regulatory obligation into a manageable, systematic process. It’s more than just a document; it’s a strategic asset designed to streamline your compliance journey, mitigate risks, and foster a robust quality management system.
The Criticality of EU MDR Essential Requirements
The EU MDR replaced the outdated Medical Device Directive (MDD), significantly elevating the bar for medical device safety and efficacy. At its core are the Essential Requirements (ERs) listed in Annex I, which cover everything from design and manufacturing to labeling and post-market surveillance. These requirements are broad yet specific, demanding a deep understanding of how they apply to each unique device. Failing to demonstrate conformity to every applicable ER is a direct impediment to achieving CE marking and, consequently, to placing your device on the European market.
Manufacturers must not only identify which requirements apply to their device but also provide robust, documented evidence of compliance. This involves cross-referencing design specifications, risk management files, clinical evaluation reports, and various technical documentation elements. Without a systematic approach, this process can quickly become fragmented, error-prone, and incredibly time-consuming, jeopardizing market timelines and potentially leading to costly regulatory actions.
Why a Dedicated Essential Requirements Checklist is Indispensable
In an environment where precision and thoroughness are non-negotiable, relying on ad-hoc methods or fragmented documentation is a recipe for disaster. A well-designed MDR compliance checklist provides a centralized, structured means to manage your conformity assessment. It brings clarity to the often-opaque regulatory language, breaking down complex requirements into actionable items. This systematic approach ensures that no critical element is overlooked, significantly reducing the risk of non-conformity during audits or market surveillance activities.
Beyond merely ticking boxes, a robust essential requirements document fosters a proactive approach to quality and safety. It empowers regulatory affairs teams, quality managers, and R&D departments to collaborate effectively, ensuring that compliance is integrated into every stage of the product lifecycle, not just as an afterthought. This integration ultimately leads to more robust device development, reduced time-to-market, and greater confidence in your product’s regulatory standing. Leveraging an Mdr Essential Requirements Checklist Template can truly transform your approach from reactive problem-solving to proactive risk mitigation and sustained excellence.
Key Elements of an Effective MDR Essential Requirements Checklist
An effective essential requirements template is more than just a list of articles; it’s a dynamic tool that supports thorough documentation and ongoing compliance. To be truly valuable, it should incorporate several key functionalities that guide users through the intricate process of demonstrating conformity. Such a template becomes a central piece of your technical documentation, facilitating audits and regulatory submissions.
Here are the essential components to look for and implement:
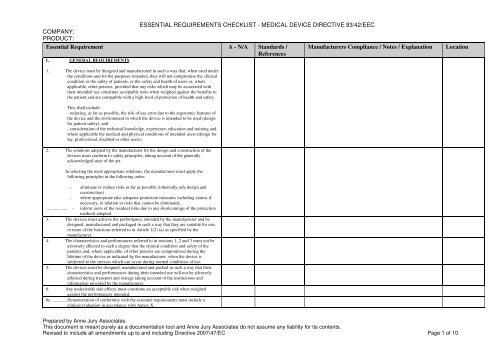
- **Clear Reference to MDR Annex I:** Each item in the checklist should directly reference the specific clause or sub-clause from Annex I of the EU MDR (e.g., ER 1, ER 9.1, ER 17.2). This ensures traceability and eliminates ambiguity.
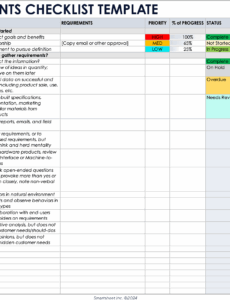
- **Applicability Assessment:** For each requirement, there should be a clear mechanism to indicate its **applicability** to your specific medical device (e.g., “Yes,” “No,” “Not Applicable”). If “No” or “Not Applicable” is selected, a concise justification should be required.
- **Compliance Status:** A dedicated field to indicate the current **status of compliance** for each applicable requirement (e.g., “In Progress,” “Pending Review,” “Completed,” “Requires Action”).
- **Evidence of Conformity:** This is perhaps the most critical section. For each applicable requirement, the template must provide space to link or reference specific **documentation that demonstrates compliance**. This could include references to risk management files, design specifications, test reports, clinical evaluation reports, usability studies, software verification and validation reports, or labeling information.
- **Traceability Matrix Integration:** Ideally, the template should serve as or integrate into a broader **traceability matrix**. This means linking essential requirements to specific design inputs, outputs, verification and validation activities, and risk controls.
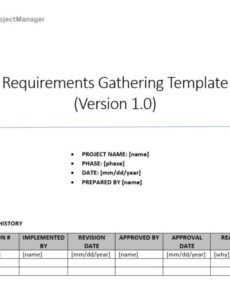
- **Responsible Party and Date:** Assigning **ownership** for each requirement (e.g., “Responsible Engineer,” “QA Manager”) and tracking the **completion date** ensures accountability and provides a clear audit trail.
- **Review and Approval Signatures:** Sections for **formal review and approval** by relevant personnel (e.g., Regulatory Affairs, Quality Assurance) are crucial to signify that the assessment has been thoroughly vetted and endorsed.
- **Version Control:** Like any critical document, the essential requirements document must include **version control** information, detailing changes, dates, and authors to maintain document integrity over time.
Streamlining Your Regulatory Journey: Practical Application
Implementing an MDR essential requirements checklist effectively requires more than just filling out a form; it demands integration into your broader quality management system (QMS) and a consistent methodology. Start by customizing the template to perfectly align with your device’s specific characteristics and your organizational structure. For example, a software-as-a-medical-device (SaMD) will have different applicability considerations than a sterile implantable device. Tailor the sections for evidence and responsible parties to reflect your internal documentation system and team roles.
Once customized, make the medical device regulation requirements template a living document. It should be introduced early in the device development process, ideally during the design input phase, and evolve alongside the device. Regular internal reviews, perhaps quarterly or at key project milestones, are vital to ensure that compliance is maintained throughout the product lifecycle, including any design changes or updates to the regulation itself. Train your teams on how to use the conformity assessment tool, emphasizing its role in demonstrating due diligence and facilitating effective communication between departments. Leverage this structured approach to prepare for external audits, using it as a roadmap to present your evidence of conformity clearly and efficiently.
Beyond Compliance: Cultivating a Culture of Quality
While the immediate goal of using an MDR Essential Requirements Checklist Template is to achieve and maintain regulatory compliance, its impact extends far beyond mere box-ticking. By instilling a disciplined, systematic approach to requirement assessment, organizations begin to cultivate a deeper culture of quality and safety. It shifts the mindset from viewing regulations as an obstacle to seeing them as a foundational framework for excellence. When every team member understands how their work contributes to fulfilling essential requirements, product development becomes more robust, risk management more proactive, and overall device quality significantly enhanced.
This proactive stance not only minimizes regulatory headaches but also strengthens market confidence in your products. A well-managed and documented conformity assessment process demonstrates a commitment to patient safety and product performance, which is invaluable in today’s competitive and highly scrutinized medical device market. The structured guidance provided by a comprehensive regulatory checklist for medical devices transforms a compliance task into an opportunity to solidify your organization’s commitment to high standards, ultimately driving innovation responsibly and sustainably.
Frequently Asked Questions
What are the EU MDR Essential Requirements?
The EU MDR Essential Requirements (ERs), detailed in Annex I of the regulation, are a comprehensive set of safety and performance requirements that medical devices must meet to be placed on the European market. They cover various aspects from device design, manufacturing, and packaging to labeling, clinical evaluation, and post-market surveillance.
Who should use an MDR compliance checklist?
An MDR compliance checklist is essential for medical device manufacturers, particularly those involved in regulatory affairs, quality assurance, research and development, and technical documentation. It’s also beneficial for consultants assisting manufacturers with EU MDR conformity assessment.
Can this template be customized for specific device types?
Absolutely. A robust MDR essential requirements checklist template is designed to be adaptable. Manufacturers should customize it to reflect the specific class, nature, and intended purpose of their medical device, allowing for clear identification of applicable requirements and relevant evidence.
How often should the essential requirements document be updated?
The essential requirements document should be considered a living document. It should be reviewed and updated regularly throughout the device’s lifecycle, especially during design changes, post-market surveillance findings, or any updates to the EU MDR or related harmonized standards. Annual reviews are a good baseline, but more frequent updates may be necessary depending on device complexity and regulatory landscape changes.
Is this template a substitute for professional regulatory advice?
No, an MDR essential requirements checklist template is a valuable tool for organizing and managing your compliance efforts, but it is not a substitute for professional regulatory advice. Medical device regulations are complex and dynamic; it’s always recommended to consult with qualified regulatory experts or Notified Bodies for specific interpretations and guidance.
In the dynamic and demanding world of medical device regulation, achieving and maintaining compliance with the EU MDR’s Essential Requirements is not merely a bureaucratic hurdle; it is a fundamental pillar of patient safety and market access. The journey through Annex I, with its intricate details and extensive evidence demands, can be daunting. However, armed with a meticulously crafted Mdr Essential Requirements Checklist Template, manufacturers gain an invaluable strategic advantage.
This powerful tool transforms complexity into clarity, providing a structured pathway to demonstrate conformity, streamline documentation, and confidently navigate audits. Embracing such a systematic approach not only mitigates significant regulatory risks but also champions a culture of uncompromising quality and continuous improvement within your organization. Invest in this critical resource, and empower your teams to build a more robust future for your medical devices, ensuring both compliance and sustained success in a highly regulated global market.