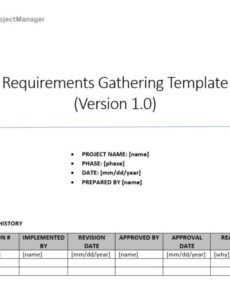
Developing a medical device is a journey fraught with complexities, demanding precision, rigorous testing, and an unwavering commitment to patient safety and regulatory compliance. At the very heart of this intricate process lies the definition of design inputs – the foundational bedrock upon which every subsequent development activity is built. These inputs translate user needs, intended uses, and regulatory mandates into clear, measurable, and verifiable specifications for the device. Without a robust and systematic approach to capturing these requirements, projects can easily derail, leading to costly delays, rework, and, most critically, devices that fail to meet user expectations or regulatory standards.
This is precisely where a well-structured Design Input Requirements Medical Device Template becomes an indispensable asset. It’s more than just a document; it’s a strategic tool that streamlines the initial phases of device development, ensuring that all critical needs and constraints are identified, documented, and approved before design and development even begin in earnest. For product managers, engineers, quality assurance specialists, and regulatory affairs professionals alike, having a standardized framework helps to mitigate risks, enhance communication across multidisciplinary teams, and lay a solid groundwork for successful product realization.
The Unshakeable Foundation: Why Design Inputs Matter So Much
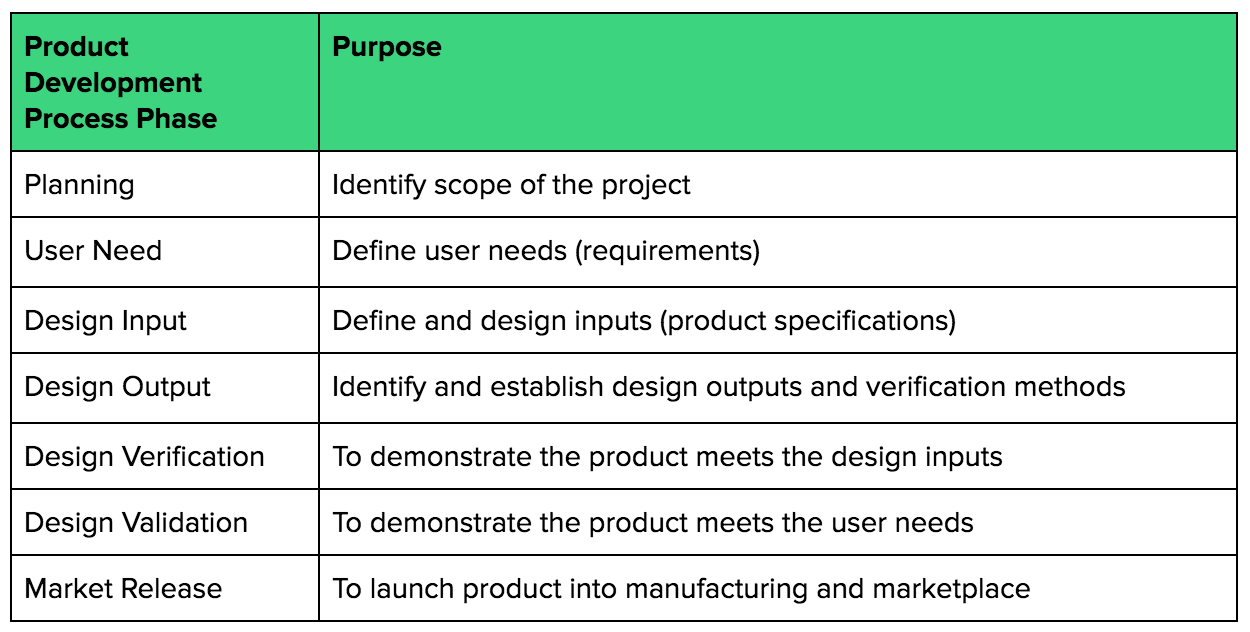
Design inputs are the comprehensive set of requirements that serve as the basis for the design and development of a medical device. They are essentially a detailed description of what the device must do, how it must perform, and what conditions it must meet to satisfy its intended use and user needs. Think of them as the blueprint before the construction begins; every wire, component, and line of code must ultimately trace back to these initial specifications.
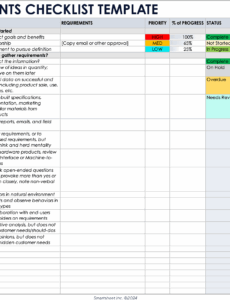
Failing to adequately define these initial requirements can have cascading negative effects throughout the entire product lifecycle. Vague or incomplete specifications can lead to misinterpretations by the design team, resulting in features that don’t align with user expectations or, worse, safety compromises. Regulatory bodies like the FDA and international standards such as ISO 13485 explicitly mandate robust design control processes, with design inputs being a critical first step. Non-compliance in this area can lead to audit findings, costly redesigns, market delays, and even product recalls.
Key Benefits of Utilizing a Standardized Medical Device Design Input Template
Adopting a structured template for capturing design inputs offers a multitude of advantages, transforming a potentially chaotic process into an organized and efficient workflow. It provides a common language and framework for all stakeholders, ensuring consistency and clarity from the outset. By front-loading the effort to define requirements rigorously, organizations can significantly reduce risks and costs downstream.
Here are some of the standout benefits:
- **Enhanced Clarity and Consistency**: A template guides stakeholders to provide complete and unambiguous information, reducing the chances of misinterpretation.
- **Streamlined Compliance**: It inherently supports regulatory requirements (e.g., 21 CFR Part 820.30 for Design Controls, ISO 13485), making audit preparation smoother.
- **Improved Communication**: By standardizing the format, it fosters better understanding and collaboration across cross-functional teams, from marketing to engineering to regulatory.
- **Risk Mitigation**: Identifying and addressing potential issues early in the design input phase prevents costly redesigns or critical failures later on.
- **Increased Efficiency**: Teams spend less time debating what information is needed and more time focusing on generating quality requirements.
- **Basis for Verification and Validation**: Well-defined device design requirements directly translate into clear criteria for testing, ensuring the final product meets its intended specifications and user needs.
- **Scalability**: A template can be adapted for various device complexities, offering a scalable solution for both simple and highly complex medical devices.
What Goes Into a Comprehensive Design Input Specification?
A comprehensive set of design input specifications typically covers several critical categories to ensure every aspect of the medical device is meticulously planned. These categories address not only the technical functionalities but also the user experience, safety, and regulatory landscape. A robust medical device design input template guides teams through capturing these diverse requirements methodically.
Consider including the following essential elements in your product design inputs for medical devices:
- **User Needs**: These describe the problems or goals that the end-user wants to achieve, expressed in the user’s language. Examples include “The device shall allow a single user to perform a blood glucose measurement within 30 seconds.”
- **Intended Use and Indications for Use**: A clear statement defining the purpose of the device, the patient population, and the conditions for which it is used.
- **Functional Requirements**: What the device must *do*. These are the core operational capabilities. For instance, “The device shall accurately measure temperature within +/- 0.1°C.”
- **Performance Requirements**: How well the device must perform its functions, often including speed, accuracy, precision, and reliability. Examples: “The battery life shall be a minimum of 8 hours of continuous use.”
- **Safety Requirements**: Specifications designed to mitigate hazards and risks, ensuring the device is safe for users and patients. This might include “The device shall shut down automatically if an over-temperature condition is detected.”
- **Regulatory and Standards Requirements**: Direct references to applicable regulations (e.g., FDA 21 CFR Part 820, EU MDR) and industry standards (e.g., ISO 13485, ISO 14971, IEC 60601).
- **Usability Requirements**: How easy and intuitive the device is to operate, including aspects like human-machine interface, display clarity, and interaction flows.
- **Environmental Requirements**: Conditions under which the device must operate, be stored, and transported (e.g., temperature, humidity, ingress protection).
- **Maintenance and Serviceability Requirements**: Specifications related to how the device will be maintained, calibrated, and serviced throughout its lifecycle.
- **Labeling and Packaging Requirements**: Details for product labeling, instructions for use (IFU), warnings, and packaging specifications.
- **Compatibility Requirements**: How the device interacts with other devices, systems, or consumables.
- **Security Requirements**: Critical for connected medical devices, specifying protection against unauthorized access or data breaches.
Implementing Your Medical Device Design Input Template: Best Practices
Having an excellent template for medical device requirements is only the first step; effective implementation is key to unlocking its full potential. To truly leverage the power of structured design input processes, teams must adopt certain best practices that foster collaboration, clarity, and continuous improvement. This approach ensures that the design controls are not just a compliance checkbox but a value-adding activity.
- Involve Cross-Functional Teams: Design input definition should not be an isolated task. Engage representatives from marketing, engineering, quality assurance, regulatory affairs, clinical, and even end-users. Diverse perspectives lead to more comprehensive and accurate requirements.
- Make Requirements Measurable and Verifiable: Each requirement should be clear, unambiguous, and, whenever possible, quantifiable. Phrases like "easy to use" should be refined into "The device shall achieve a System Usability Scale (SUS) score of 80 or higher with target users." This allows for objective verification.
- Establish Traceability: From the outset, plan how each design input will be linked to specific design outputs, verification activities, and validation activities. Traceability is crucial for demonstrating compliance and understanding the impact of changes.
- Implement a Robust Review and Approval Process: All design input specifications must undergo thorough review by relevant stakeholders and receive formal approval. This sign-off signifies agreement and commitment from the team.
- Utilize Version Control: As requirements evolve, maintaining a clear history of changes, including who made them and why, is vital. A good design control template should facilitate or integrate with a version control system.
- Prioritize and Categorize Requirements: Not all requirements have the same level of criticality. Prioritizing helps the development team focus on the most essential aspects first, especially when resources are limited. Categorization (as outlined above) helps organize the information.
Common Pitfalls to Avoid in Design Input Definition
Even with a comprehensive Design Input Requirements Medical Device Template, teams can stumble if they’re not aware of common pitfalls. Avoiding these traps can save significant time, money, and frustration during development. Proactive identification and mitigation of these issues are hallmarks of effective design control.
One frequent issue is vague or ambiguous requirements. Phrases like "the device should be user-friendly" are unhelpful because they are subjective and cannot be objectively tested. Instead, specific, measurable criteria are needed. Another pitfall is scope creep, where new requirements are added without proper evaluation or impact analysis, leading to project delays and budget overruns. A disciplined change control process is essential here.
Neglecting to adequately incorporate user needs and usability considerations can lead to devices that are technically sound but impractical or unsafe in real-world use. It’s crucial to distinguish between what the device can do and what the user needs it to do. Furthermore, a lack of clear traceability between design inputs, outputs, verification, and validation activities is a significant compliance risk and makes debugging problems incredibly difficult. Finally, overlooking non-functional requirements such as reliability, maintainability, and cybersecurity, especially for connected devices, can have severe consequences later in the product’s life cycle.
Frequently Asked Questions
What is the primary purpose of a design input document?
The primary purpose of a design input document is to clearly define the requirements for a medical device before design and development begin. It translates user needs, intended use, and regulatory mandates into measurable and verifiable specifications, serving as the foundation for the device’s design, verification, and validation activities.
How does a design input template help with FDA compliance?
A well-structured design input template directly supports FDA compliance (specifically 21 CFR Part 820.30 for Design Controls) by ensuring that all necessary requirements are systematically captured, documented, reviewed, and approved. It provides objective evidence that design inputs have been established and are adequate, which is critical for successful FDA audits and submissions.
Can a small startup benefit from using a detailed design input template?
Absolutely. While large corporations benefit from standardization, small startups often benefit even more due to limited resources. A detailed template provides a clear roadmap, prevents costly mistakes and rework, accelerates compliance, and helps articulate product vision to investors, all of which are crucial for a startup’s success.
What’s the difference between user needs and design inputs?
User needs describe the problems or goals that the end-user wants to achieve, expressed in the user’s language, often gathered through research. Design inputs are the engineering specifications derived from these user needs, translating them into technical, measurable, and verifiable requirements that the device must meet. User needs are “what the user wants”; design inputs are “what the device must do to meet those wants.”
Who is typically responsible for creating and approving design inputs?
While a cross-functional team collaborates on generating design inputs (including marketing, engineering, clinical, regulatory), the ultimate responsibility for creating and maintaining the design input document often lies with a lead engineer or product manager. Final approval typically requires sign-off from key stakeholders, including quality assurance, regulatory affairs, and senior management, to ensure alignment and commitment.
The journey from a groundbreaking idea to a fully realized, market-approved medical device is intricate, yet profoundly rewarding. At every stage, precision, documentation, and an unwavering commitment to quality are paramount. The initial phase of defining design inputs, while seemingly administrative, forms the very core of this entire endeavor, dictating the device’s capabilities, safety profile, and ultimately, its success in improving patient lives.
By embracing and effectively utilizing a comprehensive Design Input Requirements Medical Device Template, development teams are not just fulfilling a regulatory checkbox; they are actively building a robust, traceable, and quality-driven foundation for their device. This systematic approach fosters innovation within a controlled environment, minimizes costly errors, and accelerates the path to market, ensuring that the final product is not only compliant but also truly effective and safe for those who rely on it. Invest in your design inputs, and you invest in the future success and safety of your medical device.