Navigating the intricate landscape of medical device compliance can often feel like a formidable challenge. Manufacturers are tasked with demonstrating their products meet rigorous safety and performance standards, a process that demands meticulous attention to detail and an unwavering commitment to quality. The sheer volume of regulations, particularly concerning market access in regions like the European Union, can be overwhelming for even the most seasoned regulatory professionals.
In this complex environment, having a reliable, structured tool isn’t just helpful—it’s absolutely essential. This is where an Mdd Essential Requirements Checklist Template becomes an indispensable asset, transforming potential chaos into a clear, manageable pathway towards compliance. Such a template is more than just a document; it’s a strategic framework designed to guide medical device manufacturers through the critical evaluation of their products against the core safety and performance criteria required for EU market entry.
Navigating the Complex World of Medical Device Compliance
For decades, the Medical Device Directive (MDD 93/42/EEC) served as the cornerstone of regulatory compliance for medical devices placed on the European market. It laid out fundamental “Essential Requirements” that manufacturers had to meet to ensure their devices were safe, performed as intended, and did not compromise public health. While the MDD has now been largely superseded by the Medical Device Regulation (MDR (EU) 2017/745), its principles of robust safety and performance criteria endure, now articulated as “General Safety and Performance Requirements” (GSPRs) under the MDR.
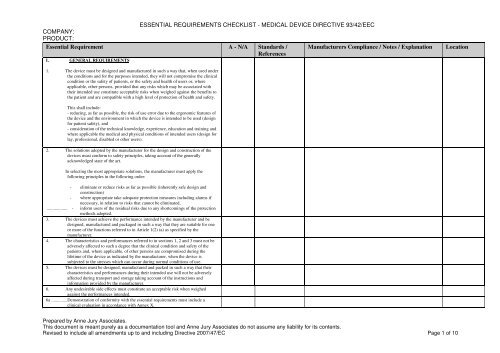
The transition from MDD to MDR has introduced an even higher bar for compliance, requiring more extensive clinical evidence, greater traceability, and enhanced post-market surveillance. However, the foundational need to systematically verify that a device addresses all applicable regulatory mandates remains paramount. This continuous need for structured assessment underscores why a well-designed essential requirements checklist, adaptable to current regulations, is a critical tool in a manufacturer’s arsenal.
Why a Dedicated Essential Requirements Checklist is Indispensable
The benefits of leveraging a specialized medical device essential requirements checklist extend far beyond mere regulatory adherence. It’s a proactive measure that imbues confidence, streamlines processes, and ultimately safeguards both patients and manufacturers.
Here are some key advantages:
- Ensured Comprehensive Coverage: A structured template ensures no critical requirement is overlooked, providing a systematic approach to assessing compliance against every applicable point of the **MDD essential requirements** or MDR GSPRs.
- Enhanced Efficiency: Rather than sifting through lengthy regulatory texts each time, a checklist offers a consolidated, digestible format. This significantly speeds up the assessment process during design and development, as well as for periodic reviews.
- Reduced Risk of Non-Compliance: By systematically identifying gaps early in the product lifecycle, manufacturers can implement corrective actions before they become costly or delay market access. This proactive risk management is invaluable.
- Improved Audit Readiness: When a notified body or regulatory authority comes knocking, having a clearly documented, well-maintained compliance checklist demonstrates a robust approach to regulatory affairs. It provides concrete evidence of due diligence.
- Facilitates Cross-Functional Collaboration: A universal checklist provides a common language and framework for teams across R&D, quality assurance, regulatory affairs, and production, ensuring everyone is aligned on the necessary compliance criteria.
- Consistent Documentation: The template standardizes how essential requirements compliance is documented, making it easier to maintain a coherent and complete technical file or design dossier, a critical part of regulatory submissions.
Key Elements of an Effective Medical Device Essential Requirements Checklist
A truly effective medical device essential requirements checklist is meticulously structured, logical, and easy to navigate. It should serve as a dynamic document, capable of guiding teams through the complex journey from concept to market and beyond. While specific elements will align with either the MDD’s Essential Requirements or the MDR’s General Safety and Performance Requirements, the underlying structure remains similar.
Typically, a robust checklist template will include sections that cover:
- General Requirements: This often includes fundamental principles related to **safety and performance**, risk management, clinical evaluation, and quality management systems.
- Design and Manufacturing Requirements: Detailed criteria concerning the materials used, design for patient safety, manufacturing processes, sterility, and stability. This section might include considerations for preventing infection or ensuring biocompatibility.
- Information Supplied by the Manufacturer: Mandates regarding labeling, instructions for use (IFU), declarations of conformity, and other critical information necessary for safe and effective use of the device. This ensures users have all the necessary guidance.
- Identification and Traceability: Requirements for unique device identification (UDI), batch codes, and other means of tracking devices throughout their lifecycle, crucial for post-market surveillance.
- Specific Requirements for Certain Devices: Tailored sections for particular types of devices, such as those that are implantable, active, or contain medicinal substances, addressing their unique risks.
- Evidence of Conformity: Space to document how each requirement is met, referencing specific sections of the technical documentation, test reports, risk analyses, or clinical data. This is where the “proof” is stored.
- Assessment Status and Remarks: Fields to indicate whether a requirement is applicable, met, partially met, or not met, along with notes for further action or justification. This provides a clear status update for each item.
A comprehensive medical device compliance checklist will be designed to be intuitive, allowing users to quickly ascertain the status of each requirement and identify areas needing further attention.
Implementing Your Essential Requirements Checklist Template: Best Practices
Merely having an **Mdd Essential Requirements Checklist Template** isn’t enough; its true value is unlocked through effective implementation and consistent use. Integrating this tool into your existing quality management system (QMS) and product development lifecycle is paramount.
Firstly, ensure the template is customized to your specific device and its classification. Not all requirements will apply to every device, so tailoring the checklist prevents unnecessary work and provides clarity. Secondly, make it a living document. It should be reviewed and updated regularly, especially in response to regulatory changes (like the full transition to MDR), new product features, or feedback from post-market surveillance. Assign clear ownership for maintaining and updating the checklist.
Thirdly, embed its use at critical stages of your product development process. Utilize it during the design input phase to ensure requirements are considered from the outset. Employ it during design reviews to verify ongoing compliance and as a final check before preparing your technical documentation for submission. Training your teams on how to effectively use the regulatory requirements template will foster a culture of compliance and consistency across the organization.
Beyond the Checklist: Continuous Compliance and Adaptation
While an Mdd Essential Requirements Checklist Template is an excellent tool for structured compliance, it’s important to view it as part of a broader, continuous effort. The regulatory landscape for medical devices is dynamic, with regulations evolving and new standards emerging. Manufacturers must embrace a mindset of continuous learning and adaptation.
Your essential requirements compliance process should not end once market approval is granted. Post-market surveillance, vigilance activities, and ongoing risk management are critical for maintaining compliance throughout the device’s entire lifecycle. The checklist should be revisited periodically, not just to confirm initial conformity, but to verify continued adherence in light of real-world performance data, complaint trends, and any updates to harmonized standards or common specifications. This proactive approach ensures your devices remain safe and effective, securing long-term market access and patient trust.
Frequently Asked Questions
What are “Essential Requirements” in the context of medical devices?
Essential Requirements were the fundamental safety and performance criteria laid out in the European Medical Device Directive (MDD 93/42/EEC). They ensured devices were safe for patients and users and achieved their intended performance. With the full implementation of the Medical Device Regulation (MDR (EU) 2017/745), these are now referred to as “General Safety and Performance Requirements” (GSPRs), which are more extensive and stringent.
Is this template still relevant with the MDR replacing the MDD?
While the MDD has been superseded by the MDR, the underlying principle of systematically evaluating a medical device against safety and performance criteria remains critically important. An effective Mdd Essential Requirements Checklist Template can be adapted or updated to become a General Safety and Performance Requirements (GSPR) checklist, ensuring its continued relevance and utility under the new regulation.
How often should I review and update my essential requirements checklist?
Your medical device compliance checklist should be reviewed periodically, at least annually, or whenever there are significant changes to your device, its intended use, manufacturing process, or relevant regulatory standards. It’s also crucial to review and update it in response to any regulatory updates, such as amendments to the MDR or changes in harmonized standards.
Can this tool help with notified body audits?
Absolutely. A meticulously maintained essential requirements checklist serves as concrete evidence of your systematic approach to regulatory compliance. During a notified body audit, presenting a comprehensive, up-to-date checklist that clearly documents how each requirement is met can significantly streamline the audit process and demonstrate your commitment to quality and safety.
The journey to bring a safe and effective medical device to market is complex, fraught with regulatory hurdles and demanding extensive documentation. Yet, with the right tools, this journey can be navigated with greater confidence and efficiency. A robust Mdd Essential Requirements Checklist Template is not merely a bureaucratic obligation; it’s a strategic asset that underpins your entire regulatory strategy, ensuring that every critical aspect of your device’s safety and performance is addressed.
Embracing such a template means adopting a proactive stance towards compliance, minimizing risks, and optimizing your path to market. It empowers your teams, standardizes your processes, and ultimately contributes to the quality and reliability of your medical devices, fostering trust with both regulatory bodies and the patients you serve. Invest in this critical tool to transform your compliance challenges into a clear, manageable, and successful endeavor.